Plant Physiology 161: 1903-1917 (2013)
Spatiotemporal seed development analysis provides insight into primary dormancy induction and evolution of the Lepidium DELAY OF GERMINATION1 Genes [W][OA]
School of Biological Sciences, Plant Molecular Science and Centre for Systems and Synthetic Biology, Royal Holloway, University of London, Egham, Surrey TW20 0EX, United Kingdom (KG, AV, GLM);
Web: 'The Seed Biology Place' - www.seedbiology.eu
University of Freiburg, Faculty of Biology, Institute for Biology II, Botany/Plant Physiology, D-79104 Freiburg, Germany (KG, AV, ABM, GLM)
Universität Osnabrück, Fachbereich Biologie, Botanik, D-49069 Osnabrück, Germany (KS, KM)
Received December 24, 2012; Accepted Feburary 19, 2013; Published Feburary 20, 2013.
DOI:10.1104/pp.112.213298
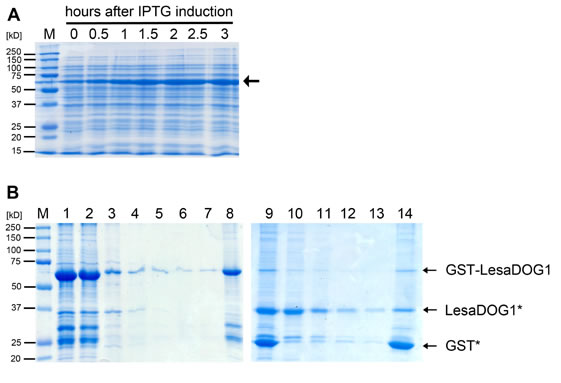
Supplemental Figure S4. Recombinant GST-LesaDOG1 fusion protein expression and purification.
A, Coomassie stained SDS-PAGE gel showing the IPTG induced expression of the 61 kDa GST-LesaDOG1 fusion protein (arrow) in E. coli. Samples of the growing bacterial culture were taken at indicated timepoints, heated in SDS-loading buffer and directly used in SDS-PAGE.
B, Coomassie stained SDS-PAGE gel showing successive fractions of second round of protein purification process (see methods for details).
1) eluted protein fraction of first purification round after dialysis;
2) matrix bound proteins after incubation of fraction 1 with Glutathion-Sepharose matrix overnight at 4 °C;
3) unbound protein;
4 -7) wash fractions, unbound protein;
8) matrix bound protein after washes;
9) matrix bound protein after TEV cleavage;
10) unbound protein after TEV cleavage;
11 -13) wash fractions, unbound protein;
14) matrix bound protein after washes.
Note: a large amount of GSTLesaDOG1 binds to matrix (2) whilst a small amount is not bound (3) and subsequently washed away (4-7); the matrix bound GST-LesaDOG1 (8) is efficiently proteolytically cleaved at its TEV recognition site resulting in LesaDOG1* appearing at ca. 37 kD and GST* at 25 kD (compare Supplemental Fig. S3) only leaving a small amount of uncut GSTLesaDOG1 (9); relatively pure LesaDOG1* is present in the unbound fractions (10-13) while GST* remains bound to the membrane (14); Fraction 10 was used in western experiments in Figs. 6 and 7 as a control and is referred to as 'DOG1'. Note the remaining presence of GSTLesaDOG1 and free GST* as detected by anti-GST antibody (Fig.6C) in fraction 10, which is hardly visible in the Coomassie stain. M, molecular mass marker.
| Article in PDF format (2 MB) Supplementary data file (4.9 MB) |
|
|
|
The Seed Biology Place |
Webdesign Gerhard Leubner 2000 |