Endosperm Weakening
Established and emerging seed model systems for endosperm weakening
Established Rosid model systems: Hormonal regulation of Brassicaceae endosperm weakening and endosperm rupture
Ethylene promotes endosperm weakening and couneracts the ABA-inhibition
Evolutionary conserved ethylene production in Brassicaceae seeds by ACC oxidase 2 (ACO2) orthologs
Hormone and seed tissue-interactions during Lepidium sativum endosperm weakening and rupture
Ethylene signalling and seed tissue-interactions during Lepidium sativum endosperm weakening and rupture
Gibberellins (GA) promotes and abscisic acid (ABA) inhibits endosperm weakening
Established Asterid model systems: Direct evidence for endosperm weakening and it's hormonal control
"Hatching enzymes" - Endosperm weakening and endosperm cell wall hydrolases
Reactive oxygen species (ROS) as a molecular mechanism of endosperm weakening and radicle growth
|
Established and emerging seed model systems for endosperm weakening |
- In the case of endosperm-limited germination, the endosperm acts as a mechanical barrier for radicle protrusion. A decline in the mechanical resistance of the micropylar endosperm (the endosperm covering the radicle tip) appears to be a prerequisite for radicle protrusion (selected reviews: Bewley, 1997, Leubner-Metzger, 2003, Kucera et al., 2005, Finch-Savage and Leubner-Metzger, 2006, Linkies et al., 2010).
- In addition to mechanical restraint, 'coat'-associated mechanisms of the endosperm, testa and/or pericarp may interfere with leaching of inhibitors of embryo elongation like ABA, or with water uptake or gas exchange, e.g. oxygen deficiency within the embryo been shown for Lepidium sativum seeds prior to testa/endosperm rupture and interferes with ethylene production by ACO (Linkies et al., 2009), the same is true for sugar beet fruits where the pericarp confers the major restraint (Hermann et al., 2007).
- Direct and indirect experimental evidence for endosperm weakening has been obtained in several species shown in the figure below. Direct evidence for endosperm weakening has been obtained by puncture-force measurement, i.e. the direct quantification of the force needed for puncturing the micropylar endosperm by a metal pin that has the shape of the radicle. Seeds of Arabidopsis or tobacco are too small for punture-force experiments. However, indirect evidence including microscopically visible early reserve breakdown in the micropylar endosperm or altered seed responses of Arabidopsis mutants and transgenic tobacco seeds, strongly supports the view that endosperm weakening is a widespread phenomenon.
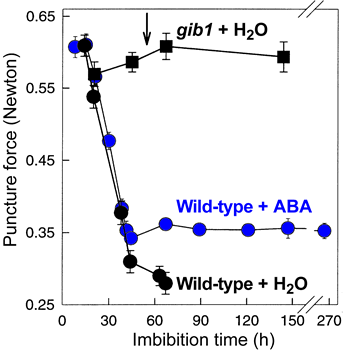
- Solanaceae species like tomato, tobacco, pepper and Datura have become model species for endosperm weakening. Puncture force measurements of tomato, including the GA-deficient gib1 mutant, demonstrated that endosperm weakening is a prerequisite to germination. Endosperm weakening is regulated by plant hormones and by environmental factors. Endosperm weakening can be promoted by GA and, at least in part, inhibited by ABA.
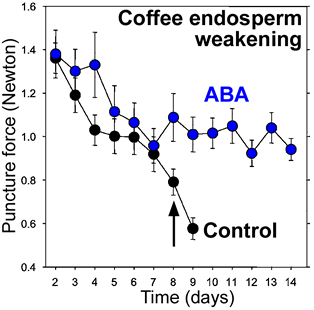
- Until 2005 direct evidence for endosperm weakening and it's promotion by GA was only obtained for the established model systems of the Asterid clade (see figure): tomato and pepper (Solanaceae), Syringa and Fraxinus (Oleaceae), coffee (Rubiaceae), and lettuce (Asteraceae). Almost nothing was known for other clades including the highly developed Rosid clade (perisperm weakening of Cucumis was known, but the hormonal regulation has not been investigated so far). Endosperm weakening of tomato (Toorop et al., 2000) and coffee (da Silva et al., 2004) appears to be biphasic with respect to abscisic acid (ABA): The first phase is not inhibited by ABA, i.e. is ABA-insensitive; but the second phase leading to radicle protrusion is inhibited by ABA.
- The publication "Endosperm-limited Brassicaceae seed germination: Abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana" by Müller, Tintelnot and Leubner-Metzger (2006) provides the first direct evidence for endosperm weakening of the Rosid clade by using seeds of Lepidium sativum, a close relative of the model plant Arabidopsis thaliana. Lepidium and Arabidopsis endosperm rupture is promoted by GA and inhibited by ABA and Lepidium endosperm weakening is promoted by GA and inhibited by ABA. These results support the hypothesis that the GA-ABA control of endosperm rupture is mediated, at least in part, by the antagonistic hormonal effects on endosperm weakening.
- The publication "Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana" by Linkies, Müller, Morris, Turecková, Wenk, Cadman, Corbineau, Strnad, Lynn, Finch-Savage, Leubner-Metzger (2009) provides direct evidence that ethylene promotes endosperm cap weakening of Lepidium sativum and endosperm rupture of both Brassicaceae species. Cross-species microarrays of the Lepidium micropylar endosperm cap and the radicle show that the ethylene-ABA antagonism involves both tissues and has the micropylar endosperm cap as a major target. Ethylene counteracts the ABA-induced inhibition without affecting seed ABA levels. The Arabidopsis loss-of-function mutants ACC oxidase2 (aco2; ethylene biosynthesis) and constitutive triple response1 (ethylene signaling) are impaired in the 1-aminocyclopropane-1-carboxylic acid (ACC)-mediated reversion of the ABA-induced inhibition of seed germination. Ethylene production by the ACC oxidase orthologs Lepidium ACO2 and Arabidopsis ACO2 appears to be a key regulatory step. Endosperm cap weakening and rupture are promoted by ethylene and inhibited by ABA to regulate germination in a process conserved across the Brassicaceae with endospermic seeds.
- The molecular mechanisms that control endosperm weakening might differ among seeds from distinct angiosperm clades. A “one-phase” ABA-inhibited endosperm weakening is evident in Lepidium sativum seeds. We speculate that during evolution the endospermic Brassicaceae seeds have retained ABA-inhibitable molecular mechanisms also found in asterid seeds (second phase of endosperm weakening, see below), whereas the ABA-insensitive phase of endosperm weakening was lost.
- Linkies et al. (2009) proposed model for the hormonal regulation of micropylar endosperm (cap) weakening and rupture in which the spatial interaction (endosperm cap and radicle) and the hormonal interaction between ABA, GA and ethylene are considered.
|
| |
|
|
|
|
|
|
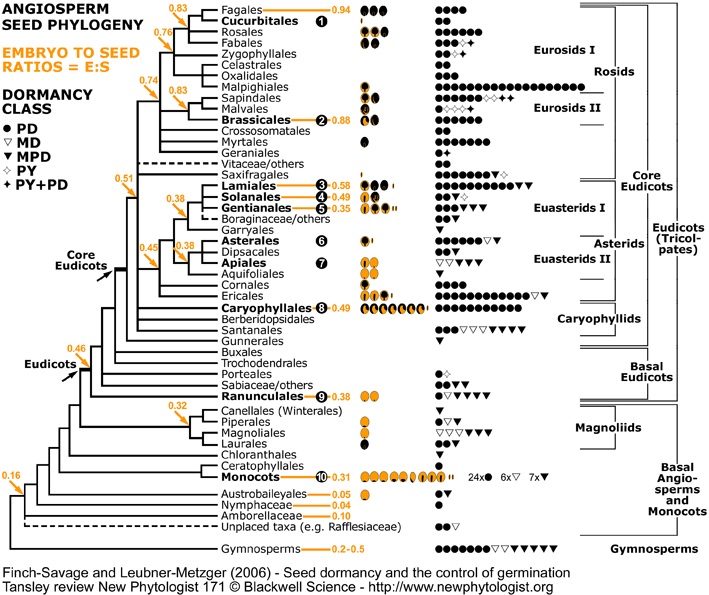
Endosperm weakening from a seed evolutionary viewpoint. Clades with experimental evidence for endosperm-limited germination and endosperm weakening are in bold and numbered (1) to (10). Puncture force measurements: C = Endosperm weakening prior to endosperm rupture; GA = Endosperm weakening prior to endosperm rupture; GA = Endosperm weakening promoted by GA; Ethylene = Endosperm weakening promoted by GA; Ethylene = Endosperm weakening promoted by ethylene; ACC = Endosperm weakening promoted by ethylene; ACC = Endosperm weakening promoted by ACC (via conversion to ethylene); ABA = Endosperm weakening promoted by ACC (via conversion to ethylene); ABA = Endosperm weakening inhibited by ABA.The experimental evidence can be summarised as follows (for references see Finch-Savage and Leubner-Metzger, 2006 and Linkies et al., 2010): = Endosperm weakening inhibited by ABA.The experimental evidence can be summarised as follows (for references see Finch-Savage and Leubner-Metzger, 2006 and Linkies et al., 2010):
Rosid clade:
(1) Cucurbitaceae: Cucumis C (perisperm weakening). (perisperm weakening).
(2) Brassicaceae: Lepidium C GA GA Ethylene Ethylene ethylene/ACC ethylene/ACC ABA ABA (Müller et al., 2006, Linkies et al., 2009), Arabidopsis. (Müller et al., 2006, Linkies et al., 2009), Arabidopsis.
Asterid clade:
(3) Oleaceae: Syringa P GA GA (Junttila, 1973), Fraxinus C (Junttila, 1973), Fraxinus C GA GA (Fig. 6, Finch-Savage & Clay, 1997). (Fig. 6, Finch-Savage & Clay, 1997).
(4) Solanaceae (Solanoideae): Lycopersicon C GA GA ABA ABA (Groot & Karssen, 1987, 1992; Toorop et al., 2000; Wu et al., 2000), Capsicum C (Groot & Karssen, 1987, 1992; Toorop et al., 2000; Wu et al., 2000), Capsicum C GA GA (Watkins & Cantliffe, 1983; Petruzzelli et al., 2003), Datura (Sanchez & Mella, 2004). (Watkins & Cantliffe, 1983; Petruzzelli et al., 2003), Datura (Sanchez & Mella, 2004).
(4) Solanaceae (Cestroideae): Nicotiana (reviewed in Leubner-Metzger, 2003), Petunia (Petruzzelli et al., 2003).
(5) Rubiaceae: Coffea C GA GA ABA ABA (da Silva et al., 2004; da Silva et al., 2005). (da Silva et al., 2004; da Silva et al., 2005).
(6) Asteraceae: Lactuca C GA GA (Tao & Khan, 1979). (Tao & Khan, 1979).
(7) Apiaceae: Apium (Jacobsen & Pressman, 1979).
Other clades:
(8) Amaranthaceae: Chenopodium (Karssen, 1976).
(9) Ranunculaceae: Trollius (Hepher & Roberts, 1985).
(10) Iridaceae: Iris C (Blumenthal et al., 1986), Poaceae: Triticum C (Blumenthal et al., 1986), Poaceae: Triticum C (Benech-Arnold, 2004). (Benech-Arnold, 2004).
Angiosperm seed evolution depicted in a phylogenetic tree of the Angiosperm Phylogeny Group II (2003). For each family, pictographs of the seed type and symbols for the dormancy class are placed next to the corresponding clade (see figure above for symbols and data source). The number of pictographs or symbols is equal to the number of families with the corresponding seed type or dormancy class, respectively. Numbers in the phylogenetic tree represent "embryo to seed" (E:S) ratios expressed as GSL (generalized least squares) values from Forbis et al. (2002, personal communication). Phylogenetic tree modified from Judd et al. (2002); Angiosperm Phylogeny Group (2003); Soltis and Soltis (2003); Stevens (2006). Figure published in the
Tansley review "Seed dormancy and the control of germination" by Finch-Savage WE and Leubner-Metzger G (New Phytologist 171: 2006). A PDF file of this review is available for download from
either "The Seed Biology Place" (Finch-Savage and Leubner-Metzger, 2006)
or the New Phytologist Trust website at New Phytologist Trust - Tansley Reviews - © Blackwell Science -
http://www.blackwell-science.com/
|
|
| |

|
| |
|
|
|
| |
|
|
|
Established Rosid model systems for endosperm weakening: Hormonal regulation of Brassicaceae endosperm weakening and endosperm rupture -
GA promotes and ABA inhibits endosperm weakening and rupture of Lepidium sativum (garden cress) |
| |
|
|
|
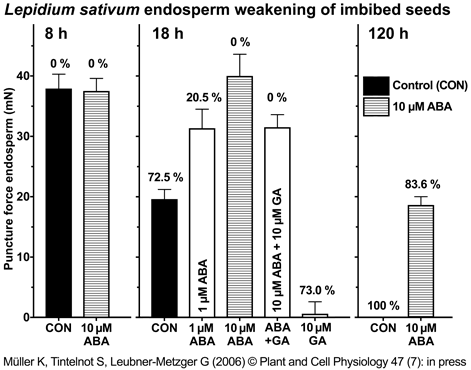
|
Direct measurement of Lepidium sativum endosperm weakening of imbibed seeds by the puncture force method.
Micropylar seed halves were dissected from whole seeds imbibed (continuous light, 18 °C) for 8 h, 18 h or 120 h without (control; CON) or with hormones (ABA and/or GA4+7 in the concentrations indicated) added to the medium. Mean values ± SE of at least 40 endosperm caps are presented. Note that all these seeds had completed testa rupture, but had intact endosperm. The numbers above the columns represent the percentage of seeds with ruptured endosperm in the corresponding seed populations.
Müller et al. (2006) |
|
The publications "Endosperm-limited Brassicaceae seed germination: Abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (garden cress) and endosperm rupture of cress and Arabidopsis thaliana" by Müller et al. (2006) and "Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana" by Linkies et al. (2009) provided the first direct evidence for endosperm weakening of the Rosid clade by using seeds of Lepidium sativum, a close relative of the model plant Arabidopsis thaliana.
Lepidium and Arabidopsis endosperm rupture is promoted by GA and ethylene, and inhibited by ABA and that Lepidium endosperm weakening is promoted by GA and etyhlene, and inhibited by ABA. These results support the hypothesis that the GA-ABA and ethylene-ABA control of endosperm rupture is mediated, at least in part, by the antagonistic effects on endosperm weakening. The molecular mechanisms that control endosperm weakening might differ among seeds from distinct angiosperm clades. A “one-phase” ABA-inhibited endosperm weakening is evident in Lepidium seeds. We speculate that during evolution the endospermic Brassicaceae (Rosid clade) seeds have retained ABA-inhibitable molecular mechanisms also found in Asterid seeds (second phase of endosperm weakening), whereas the ABA-insensitive phase of endosperm weakening was lost. Lepidium, a close relative of Arabidopsis, is an established Rosid seed model system for endosperm weakening. The complementary advantages of both systems will be exploited in future experiments to investigate the molecular mechanisms of endosperm weakening.
|
|
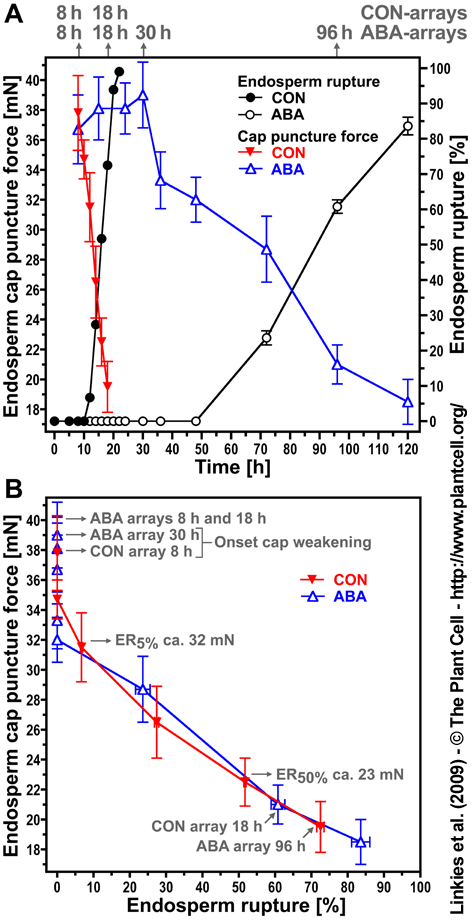 |
Figure 1. Time course of endosperm cap weakening and endosperm rupture of Lepidium sativum FR1 and the effect of abscisic acid (ABA).
(A) Endosperm cap weakening and rupture of seeds incubated without (CON) or with 10 µM ABA added to the medium in continuous light at 18°C. Endosperm cap weakening was determined by measuring tissue resistance by the puncture force method at the times indicated. The time points (after the start of imbibition) at which tissues were dissected for the microarrays are indicated in gray. All seeds selected for the puncture force measurements and for the microarrays had completed testa rupture but intact endosperm caps. ABA did not affect testa rupture.
(B) The CON and ABA seed populations showed a very similar relationship between decreasing endosperm cap puncture force and increasing percentage of seeds showing endosperm rupture. Mean values ± SE of 3 x 50 seeds for the endosperm rupture [%] and at least 50 endosperm caps for the puncture force [mN] measurements are presented.
Figure from Linkies et al. (2009) |
|
Lepidium and Arabidopsis endosperm rupture is promoted by ethylene and inhibited by ABA and that Lepidium sativum endosperm weakening is promoted by ethylene and inhibited by ABA. These results support the hypothesis that the ethylene-ABA control of endosperm rupture is mediated, at least in part, by the antagonistic effects on endosperm weakening. ACC (1-aminocyclopropane-1-carboxylic acid), the direct biosynthetic precursor of ethylene, also promotes endosperm weakening and supports the importance of the enzyme ACO (ACC oxidase), especially the CO2 isoform. Ethylene biosynthesis and signaling are important for the promoting effect and the counteraction of the ABA inhibition is mediated via interference of ethylene with ABA signaling, without affecting ABA contents (Linkies et al., 2009). The Arabidopsis loss-of-function mutants for ACC oxidase2 (aco2; ethylene biosynthesis) and constitutive triple response1 (ctr1; ethylene signaling) are impaired in the ACC-mediated reversion of the ABA-induced inhibition of seed germination (see Fig. 6 and Fig. S2 in Linkies et al., 2009). In agreement with indirect evidence for endosperm weakening Arabidopsis thaliana (Bethke et al. 2007) and Sisymbrium officinale (Iglesias-Fernandez and Matilla, 2010) this supports an evolutionary conserved mechanism for the regulation of Brassicaceae endosperm weakening. Lepidium sativum is therefore a Brassicaceae model system for studying endosperm weakening and seed-tissue interactions during germinationts (Linkies et al., 2009).
|
|
Established Rosid model systems for endosperm weakening: Hormonal regulation of Brassicaceae endosperm weakening and endosperm rupture -
Ethylene and its direct precursor ACC promotes and ABA inhibits endosperm weakening and rupture of Lepidium sativum (garden cress) |
| |
|
|
|
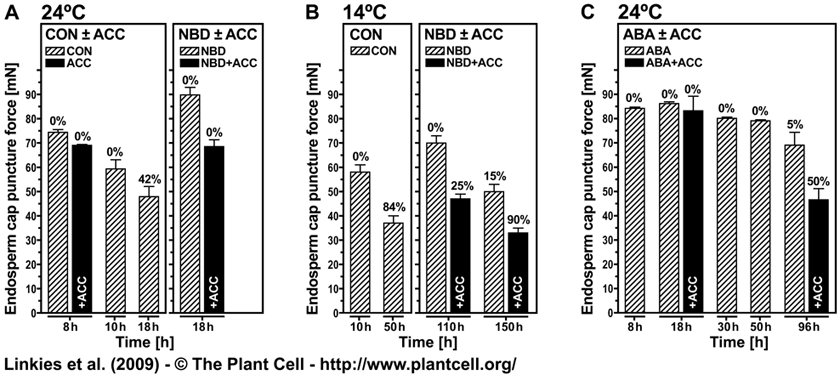
The ethylene action inhibitor 2,5-norbornadiene (NBD) binds specifically to ethylene receptors in direct competition with ethylene for the ethylene binding site. The NBD experiments therefore demonstrate that ethylene signaling via the classical ethylene-receptor-CTR1 signaling pathway is important for the promotion of endosperm weakening and for counteracting the ABA inhibition on Lepidium sativum endosperm weakening (Linkies et al., 2009). That this is an evolutionary conserved mechanism is in full agreement with results obtained with the Arabidopsis loss-of-function mutants for ACC oxidase2 (aco2; ethylene biosynthesis) and constitutive triple response1 (ctr1; ethylene signaling) are impaired in the ACC-mediated reversion of the ABA-induced inhibition of seed germination (see Fig. 6 and Fig. S2 in Linkies et al., 2009).
|
Figure 9. The effect of ACC, NBD, ABA, or their combinations on endosperm cap weakening and endosperm rupture of Lepidium sativum FR14. Linkies et al. (2009)
Endosperm cap weakening (columns) and rupture (percentages above columns) of seeds incubated without (CON) or with 1 mM ACC or 10 µM ABA added to the medium in continuous light at 24°C ([A] and [C]) or 14°C (B). Endosperm cap weakening was determined by measuring tissue resistance by the puncture force method at the times indicated. All the seeds selected for the puncture force measurements had completed testa rupture but still had intact endosperm caps; ACC, NBD, and ABA did not appreciably affect testa rupture. Mean values ±SE of 3 x 50 seeds for the endosperm rupture and at least 50 endosperm caps for the puncture force measurements are presented. Note that the puncture force values measured for the endosperm cap weakening of L. sativum FR14 were approximately twofold compared with L. sativum FR1.These higher values are accession-specific absolute differences, but the relative decreases of the two accessions are similar.
|
|
|

|
| |
|
|
|
| |
|
|
|
Evolutionary conserved ethylene production in Brassicaceae seeds by ACC oxidase 2 (ACO2) orthologs
The Arabidopsis loss-of-function mutants for ACC oxidase2 (aco2; ethylene biosynthesis) is impaired in the ACC-mediated reversion of the ABA-induced inhibition of seed germination. Ethylene production by the ACC oxidase 2 orthologs LesaACO2 (Lepidium sativum), AtACO2 (Arabidopsis thaliana) and SoACO2 (Sisymbrium officinale) appears to be a key regulatory step for endosperm cap weakening to counteract the ABA inhibition. This ethylene propduction is mainly generated by the radicle and, beyond these three Brassicaceae species with endospermic seeds, this regulation of seed germination by ACO2 orthologs is appears to be evolutionary conserved across the Brassicaceae (see Fig. S3 in Linkies et al., 2009), and beyond in Fabaceae (see pea), Solanaceae (see tobacco) and Amarathaceae (see sugarbeet). Increased ethylene production in seeds is regulated, at least in part by increased ACO gene expression and enzyme activity accumulation.
Hormone and seed tissue-interactions during Lepidium sativum endosperm weakening and rupture
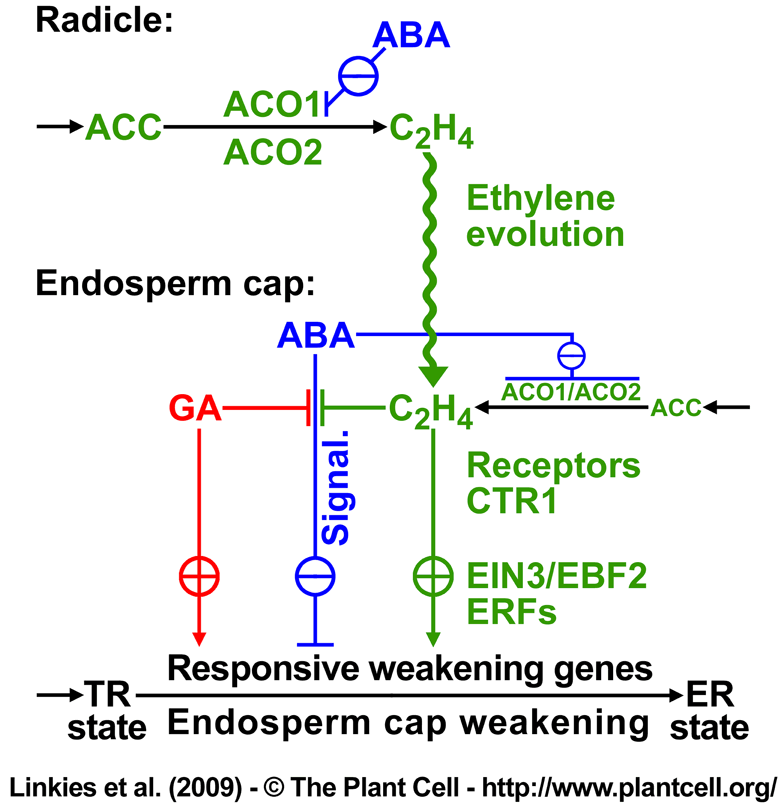
Figure 10. Proposed model for the hormonal regulation of endosperm cap weakening and rupture(Linkies et al. 2009).
According to our working model, endosperm cap weakening is required during the transition from testa rupture (TR) to endosperm rupture (ER), and endosperm cap weakening in turn requires ethylene biosynthesis and signaling. Endosperm cap weakening is a developmental process that is regulated by interactions between the cap and the radicle. GA, as an embryo signal, releases coat dormancy (if present) and induces the cap weakening process. Thereafter, weakening is a cap-autonomous process, and the rate of this process is regulated by the GA-ABA and ethylene-ABA antagonisms. The involvement of GA as antagonist of ABA in seed germination is well established in different Brassicaceae (e.g., Yamaguchi et al., 2001; Ogawa et al., 2003; Chiwocha et al., 2005; Müller et al., 2006; Iglesias-Fernandez and Matilla, 2009).
GA biosynthesis may be more important in the early phase of germination, and ethylene biosynthesis may be more important for the late phase of the process. Ethylene biosynthesis in germinating seeds is regulated differently in the radicle and the endosperm cap, and this effect is mediated by ACO2 gene expression in the following way: although both ACO2 and ACO1 are active in the radicle and endosperm cap, the total activity is greater in the former. The radicle produces ethylene in excess, and this is targeted at the endosperm cap. ABA delays the ACO activity in the radicle and inhibits ACO1 transcript accumulation, but ABA does not inhibit ACO2 transcript accumulation. The later increase in ACO activity in the radicle of ABA-treated seeds is therefore due to ACO2, and the ethylene produced promotes endosperm cap weakening by antagonizing the ABA inhibition. In the endosperm cap, ABA inhibits ACO2 and ACO1 transcript accumulation. A basal level of ethylene signaling is required to maintain ACO2 transcript levels in seeds.
Ethylene does not affect the seed ABA levels and therefore must counteract the ABA-induced inhibition of endosperm rupture by interfering with ABA signaling. High degrees of GA and ethylene sensitivity of the endosperm cap are associated with the after-ripened (post coat dormancy) seed state and are prerequisites for ethylene-enhanced expression of ABA-inhibitable downstream weakening genes in the cap. The products of the weakening genes cause endosperm cap weakening by cell wall loosening and cell separation that finally leads to endosperm rupture and radicle emergence.
The Arabidopsis loss-of-function mutants for ACC oxidase2 (aco2; ethylene biosynthesis) and constitutive triple response1 (ctr1; ethylene signaling) are impaired in the ACC-mediated reversion of the ABA-induced inhibition of seed germination. Ethylene production by the ACC oxidase orthologs Lepidium ACO2 and Arabidopsis ACO2 appears to be a key regulatory step. Endosperm cap weakening and rupture are promoted by ethylene and inhibited by ABA to regulate germination in a process conserved across the Brassicaceae.
Ethylene signalling and seed tissue-interactions during Lepidium sativum endosperm weakening and rupture
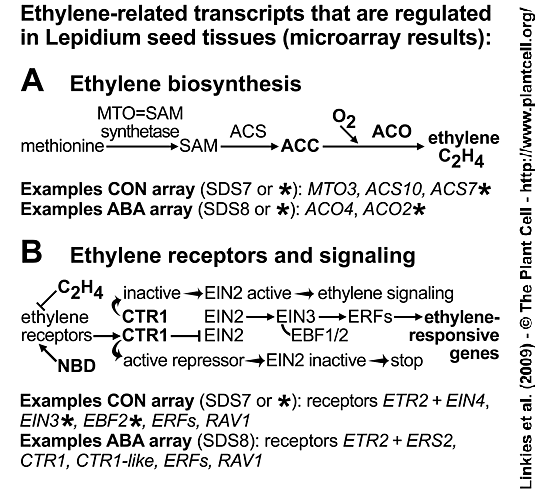
Ethylene-related regulated transcripts in Lepidium sativum FR1 seed tissues. See Figure 4 in Linkies et al. 2009.
(A) Key steps in ethylene biosynthesis include the oxygen-requiring conversion of ACC to ethylene by ACO.
(B) Key steps in ethylene signaling include ethylene binding to the receptors which can be blocked by the ethylene action inhibitor NBD (2,5-norbornadiene). In the absence of ethylene or with NBD bound the ethylene receptors activate CTR1, a negative regulator of the downstream signaling pathway and the ethylene responses are blocked. Upon ethylene binding the receptors and consequently CTR1 are inactive and the downstream signaling pathway factors (EIN2, EIN3, ERFs) become active and mediate the expression of genes that facilitate the ethylene responses.
Our findings above in both species (Arabidopsis thaliana and Lepidium sativum) suggest that ethylene sensitivity increases over time in response to ABA. This is further supported by the observation that transcript levels of the ethylene receptor ETR2 and of the negative regulator of ethylene responses LesaCTR1 (the Lepidium cDNA was cloned and qRT-PCR performed) decreased upon ABA treatment of Lepidium endosperm caps and radicle (Suppl. Figure 4B and 4D). This decrease in LesaCTR1 transcript levels suggests enhanced ethylene signaling via downstream positive factors:
Ethylene Insensitive2 (EIN2) ==> Ethylene Insensitive3 (EIN3) ==> ethylene-responsive element-binding transcription factors (ERFs)
Accumulation of EIN3 Binding F-Box2 (EBF2) transcripts (CON) and the inhibition of this accumulation by ABA (Suppl. Figure 4C) is further evidence for enhanced ethylene signaling. In the absence of ethylene, EBF2 targets EIN3 for degradation and this EBF2-EIN3 interaction is inhibited in the presence of ethylene. EBF2 is known to accumulate during ethylene signaling to prevent excess accumulation of EIN3. The ABA inhibition of the EBF2 accumulation (Suppl. Figure 4C) therefore suggests that there is no excess of EIN3 to trigger this feedback loop. Downstream of EIN3, transcripts of 6 (CON-arrays) and 18 (ABA-arrays) ERF as well as 'Related to ABI3/VP1' (RAV1, an AP2/EREBP-type transcription factor with ABI3/VP1-like domain) transcription factors of the AP2/EREBP family are significantly regulated and may therefore mediate the expression of ethylene responsive downstream genes.
|
 |
 |
|
|

  |
Grown up seedlings of Lepidium sativum (garden cress) |
|
|
| |
|
|
|

|
| |
|
|
|
| |
|
|
|
Gibberellins (GA) promotes and abscisic acid (ABA) inhibits endosperm weakening |
| |
|
|
|
|
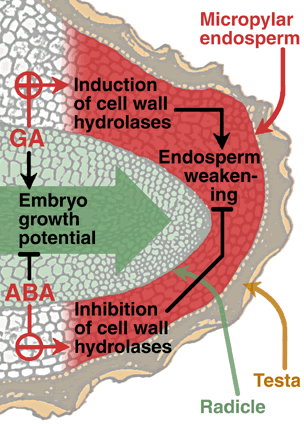
The model shows the micropylar endosperm and the radicle tip of a tobacco seed.
Gibberellins (GA) promote the induction of cell wall hydrolases and thereby promote endosperm weakening and endosperm rupture.
Abscisic acid (ABA) inhibits the induction of cell wall hydrolases and thereby inhibits endosperm weakening and endosperm rupture.
GA promotes and ABA inhibits the embryo growth potential.
We propose that endosperm weakening involves several cell-wall hydrolases and other molecular mechanisms like ROS. |
| |
|
|
|
| |
|
|
|
Established Asterid model systems: Direct evidence for endosperm weakening and it's hormonal control -
Two-phase endosperm weakening of tomato and coffee -
ABA inhibits the second phase |
| |
|
|
|
Endosperm weakening of tomato (left, Solanaceae, Asterid clade) and coffee (right, Rubiaceae, Asterid clade) |
|
| |
|
|
|
Tomato: The first phase is not inhibited by ABA and involves endo-ß-mannanase-mediated weakening. endo-ß-Mannanase in the endosperm cap is not inhibited by ABA. The second phase is inhibited by ABA and correlates with the expression of class I ß-1,3-glucanase in the micropylar cap of tomato. The second step of the biphasic endosperm cap weakening that mediates tomato seed germination is under control of ABA (Toorop et al., J Exp Bot 51: 1371-1379, 2000)
Coffee: ABA controls embryo growth potential and endosperm cap weakening during coffee seed germination. ABA inhibits a second phase of coffee endosperm weakening. endo-ß-Mannanase in the endosperm cap is inhibited by ABA (da Silva et al., Planta 220: 251-261 (2004).
|
|
| |
|
|
|
| |
|
|
|

|
| |
|
|
|
| |
|
|
|
"Hatching enzymes" - Endosperm weakening and endosperm cell wall hydrolases |
- The "hatching enzyme" hypothesis in seed biology: Ikuma and Thiman (Plant & Cell Physiol 4: 169-185, 1963) first proposed proposed for lettuce that "... the final step in the germination control process is the production of an enzyme whose action enables the tip of the radicle to penetrate through the coat".
- Endo-ß-mannanase (excellent review by Bewley, 1997) and endo-ß-1,3-glucanase were proposed to be the cause of endosperm weakening.
- In addition numerous other cell-wall modifying proteins, e.g. ß-mannosidase, a-galactosidase, cellulase, pectin methylesterase, polygalacturonase, xyloglucan endo-transglycosylase, chitinase, peroxidase and expansin were examined. Several of these studies provided evidence for the possible contribution of a specific cell wall hydrolase in a certain species and unraveled some of the complexity of the hormonal regulation of seed germination and dormancy. However, conclusive evidence for a single "germination enzyme" has not yet been found.
- Endosperm weakening and hydrolase action in lettuce, tomato, tobacco, Datura seeds seems to be promoted by gibberellins (GA) and red light (via phytochrome) and inhibited by far-red light and often by abscisic acid (ABA).
- While endo-ß-mannanase appears to be necessary for tomato endosperm weakening, it is not sufficient for the completion of germination (e.g. Toorop et al., Planta 200, 153-158, 1996; Wu et al., 2001). Abscisic acid (ABA) clearly controls the final step of radicle protrusion, but it does not inhibit tomato endosperm weakening caused by endo-ß-mannanase (e.g. Toorop et al., J Exptl Bot 51, 1371-1379, 2000; Wu et al., 2001).
- A two-phase endosperm weakening process was proposed for tomato by Toorop et al. (2000). The first phase is not inhibited by ABA and involves endo-ß-mannanase-mediated weakening. The second phase is inhibited by ABA and correlates with the expression of class I ß-1,3-glucanase in the micropylar cap of tomato.
- Tobacco seeds are too small for puncture force experiments. We proposed that in the case of tobacco, class I ß-1,3-glucanase, which is induced in the micropylar endosperm just prior to its rupture and is tightly linked with altered endosperm rupture in response to light, gibberellins (GA), ABA, and ethylene, is involved in regulating endosperm weakening (Leubner-Metzger and Meins, 1999).
- Considerable evidence from work with transgenic seeds suggests that ß-1,3-glucanase substantially contributes to the regulation of endosperm rupture, dormancy release and after-ripening of dicot seeds (Leubner-Metzger and Meins 2000; Leubner-Metzger 2002; Leubner-Metzger 2003).
- Proteomic analysis of Arabidopsis showed that ß-1,3-glucanases are among the group of glycosylphosphatidylinositol-anchored membrane proteins (GPI-APs, Elortza et al. 2003). GPI-APs are proposed to be involved as enzymes and receptors in cell adhesion, cell separation and differentiation processes. This could be a new function of ß-1,3-glucanases during endosperm weakening. In sea urchins ß-1,3-glucanases are reported as part of the embryo hatching enzyme (Bachman and McClay 1996).
- ABA inhibition of ß-1,3-glucanase in the endosperm is widespread among Solanaceae seeds (Petruzelli et al. 2003).
- ABA inhibition of ß-1,3-glucanase is also evident in perisperm weakening of Cucurbitaceae seeds where a callose layer contributes to the semipermeability of the perisperm (Yim and Bradford 1998, Amritphale et al. 2005, Ramakrishna and Amritphale 2005).
- Taken together, these findings support the view that germination control by the seed covering layers is achieved by the collaborative or successive action of several cell-wall-modifying proteins and by several different molecular mechanisms (Leubner-Metzger 2003).
|

|
| |
|
|
|
| |
|
|
|
Reactive oxygen species (ROS) as a molecular mechanism of endosperm weakening and radicle growth
|
| |
|
|
|
| See the webpage of Kerstin Müller for detailed information on the ROS project. |
|
|
|
| |
|
|
|
 |
 |
 |
 |
|
 |
| |
|
|
|

|
 |

